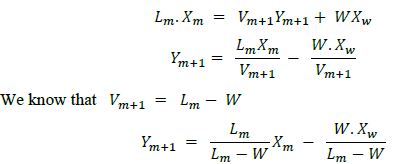Fractionation Distillation column blog contains
- Fractionation Distillation
- Fractionation column operation
- Material Balance
- Feed Plate & Feed line
- Total Reflux (Infinite Reflux Ration)
- Minimum Reflux Ratio
- Optimum Reflux Ratio
Fractionating distillation
A fractionating column a cylindrical shell divided into two sections by a series of perforated trays a reboiler and condenser.
A liquid mixture to be separated is introduced in the cylindrical column more or less centrally.
The column itself is divided into two section rectifying and stripping section.
The section above the feed plate or tray is called the rectifying section, where in vapour is washed to remove the less volatile component with the liquid return to the column from top, the portion below the feed plate including feed plates is called the stripping section.
Where in liquid is used stripping of more volatile component by using vapour perforated trays are nothing but gas liquid containing device on which gas and liquid are come into intimate contact of mass transfer to occur .
Vapors are generated in a reboiler and are feed to the bottom of the column. The liquid removed from the fractionators rich in less volatile component is called the bottom product. temperature is maximum at the bottom and minimum at the top.
The part of the condensed liquid returning to the top column is called reflux.
Why reflux is important?
When vapor reaches up side, it cool down and travel back to bottom section ( Stripping section).
When cooled reflux is added it reduces top section (rectifying section) temperature and creates temperature gradient which cool down non product components. and allow to pass only product vapour.
Temperature Gradient, energy exchange & Importance of reflux
Distillation of fluid mixture A & B is fed to any tray or reboiler.
Fluid A Boiling point 80℃ Fluid B Boiling point 85℃
[ Boiling temperature is depending upon concentration of individual components concentration.
When fluid mixture fed to column heated vapour temperature it has 90℃ If we supply steam for 85℃ then vapour will not reach up to top section. It is not possible to supply heat to all section so these vapour mixture will act as heating media.
Both Fluid A & B start vaporizing and travelling to upper section.
When vapour reach at some trays above it starts cool down and fluid B partially start condensing. In this condensation Minor portion of fluid A will also condensate.
Above this tray same phenomena will occur but the difference is Fluid A concentration will also increases
When vapour goes out from top section to condenser minor portion of Fluid B will be present in product.
So, how we can reduce concentration of Fluid B in product?
Answer - We have to retain fluid B in column by reducing some top section temperature.
So when we add cooled distillate product back to column top sections tray will be reduced and fluid B will be condensate there more. [Initially column run on full reflux]
This tray has now reduced temperature and when this fluid (with reduced temperature) goes below through downcomer it will also reduce temperature of bottom tray.
Now we have energy scenario that
- Vapour coming from bottom has high temperature.
- Reflux coming from top has low temperature.
This creates temperature gradient like this. now if we still not get purity of product and high concentration of fluid B then what we will do?
We will increase reflux ratio.
When we increases reflux ratio then it will cool top section and more fluid B will be trapped in column and result in more purity of product.
When we are increasing reflux it is also increasing in more steam consumption.
Fractionation column operation
Here is f0eed is introduced more or less centrally into a vertical of stapes.
Vaporizing in the section above the feed is worked with liquid to remove or absorb the less voluble component since no extraness material is added as in the case of absorption, the washing liquid in the case is provided by condensing the vapour from the top which is rich in more.
The liquid returned to and the material permanently removed is the distillation which may be vapour or liquid, rich in more voluble component. In the section below the feed the liquid is stripped of voluble component by vapour produced at the bottom by partial vaporization of the bottom liquid in the reboiler.
The liquid removed rich in less voluble component are the residue bottoms.
Material Balance
Rectifying Section:-
Point –
V = L + D
L = V – D
D = V – L
Vapour flow plate n = liquid flow from plate + distillate withdraw
Feed Plate & Feed line
Total Reflux (Infinite Reflux Ratio)
In this case enough material is charged to distillation assembly and column is operated under total reflux. During the operation of the column vapour issuing from top are condensed and all condensed stream fed back to the column as reflux.
Also all liquid going to the reboiler is vaporized and is fed to the column.
Thus under condition of total reflux F = 0, D = 0, and W = 0 therefore throughout the column.
The slope (L/V) both the operating lines becomes unity and hence both the operating lines coincide with the diagonal and minimum number degree of separation .
Total reflux operation corresponds to maximum reboliler heat supply and condenser cooling capacity for the separation.
Minimum Reflux Ratio
A minimum reflux ratio is that ratio at which infinite number of phase is required for desired separation.
At minimum reflux ratio, required heat supply for reboiler and coolant supply for condenser are minimum.
Optimum Reflux Ratio
Any reflux ratio between infinite reflux ratio requiring minimum number of plates and minimum reflux requiring infinite number of plates is a workable system which requires finite stages for desired degree of separation.
At minimum reflux ratio as infinite number of plates required fixed cost is also infinite while cost of heat supply to reboiler and condenser coolant is minimum.
As the reflux ratio is increased, the number of plates decreases and the fixed cost decreases at first passes through a minimum and then increases as with higher reflux ratio the diameter of the column and sizes of reboiler and condenser increases.
The operating cost increases continuously as reflux ratio is increases as it is directly proportional to (R+ 1). At total reflux through the number supply to reboiler and condenser coolant is maximum and also large capacity reboiler and condenser are needed.
The total cost which is sum of fixed cost and operating cost also decreases to a minimum and then increases with reflux ratio occurs at a point where the sum of the fixed cost and operating cost is a minimum, as rough approximation.
The optimum reflux ratio usually lies in the range of 1.1 to 1.5 times the minimum reflux
[ads id="ads1"]
Feed Plate & Feed Line
The plate on which feed is introduced is called feed plate. The feed to column may be introduced as:
(a) Cold liquid
(b) Liquid at its bubble point (saturated liquid)
(c) Partially vaporized (partially vapour and partially liquid)
(d) Saturated vapour at its dew point and
(e) Super heated vapour.
The condition of feed introduced on feed plate alters the phase flow rates. The phase flow rates are shown based on the fact the column liquid s always its bubble point and vapour is always at its dew point.
To calculate the change in phase flow rates by introduction of feed factor ‘q’ is introduced. The q is a measure of thermal condition of feed and is defined as the number of moles of saturated liquid resulting in stripping section for each mole of feed introduced.